About
Study purpose
The respiratory virus surveillance study, funded by the Centers for Disease Control and Prevention, aimed to measure how much and what types of viral respiratory illness occurred in the Navajo Nation, White Mountain Apache Tribal Lands, and in Alaska from 2019-2025. This information helps to know the best ways to prevent illness.
Study activities
We enrolled children younger than 5 years of age who were hospitalized (inpatients) or had an outpatient visit (for example, a clinic visit or visit to urgent care) with a respiratory infection. Some study locations also enrolled adults. The study took place at health facilities in Navajo Nation (Chinle, Fort Defiance, Gallup, Tuba City), the White Mountain Apache Tribal lands, and Alaska (Anchorage and Yukon-Kuskokwim Delta), and participation was voluntary.
Following informed consent, we asked about the patient’s household and their illness symptoms, and we documented medical conditions and information about the illness. In addition, we collected a nasal swab to test for respiratory viruses including respiratory syncytial virus (RSV), SARS-CoV-2 (the virus that causes COVID-19), influenza, and human metapneumovirus (HMPV).
What we learned
- In the first year of the study, 2019-2020, the rates of RSV hospitalization among American Indian and Alaska Native (AI/AN) children under 2 years were 3 to 8 times higher than US national rates.
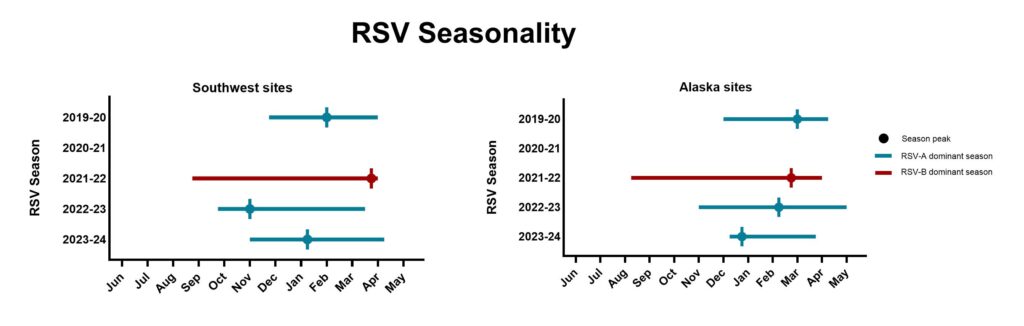
- In general, RSV circulated from winter (November/December) to spring (April/May). Circulation was interrupted during the COVID pandemic.
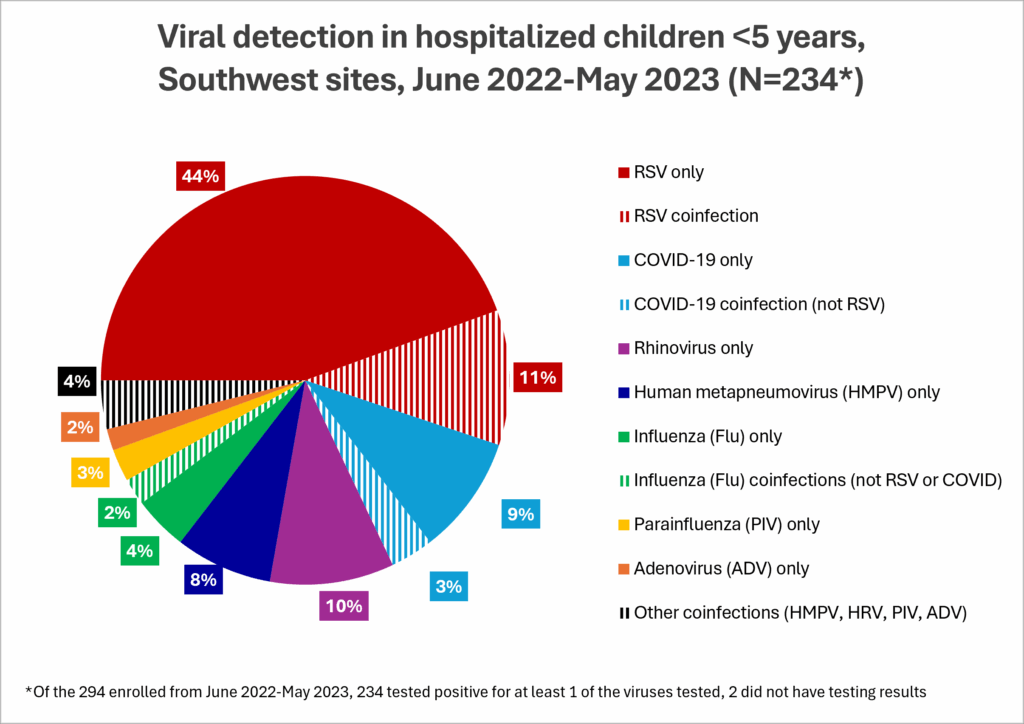
- Before RSV prevention products were available, we found that more than half of AI/AN children hospitalized for respiratory illness in the study had RSV. Other common viruses found in hospitalized children included COVID-19, rhinovirus, HMPV, and influenza (or “flu”).
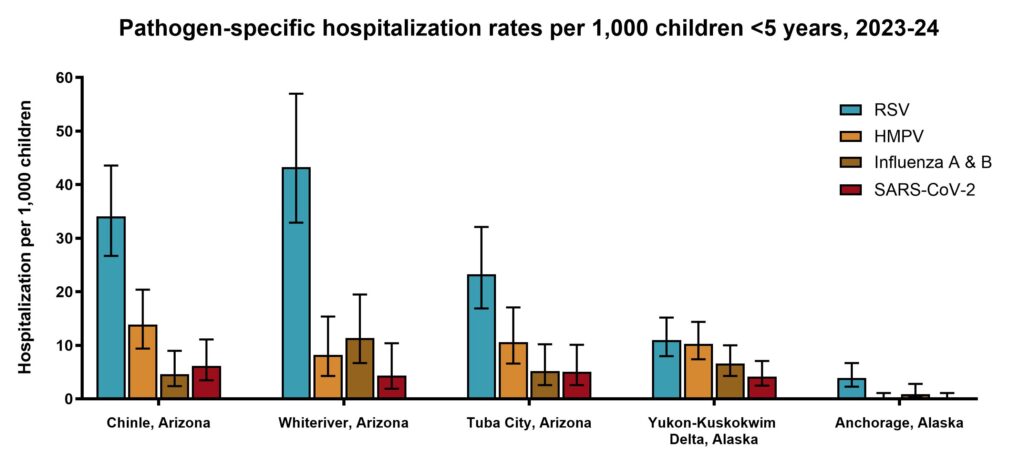
- The RSV monoclonal antibody, nirsevimab, is highly effective in preventing RSV hospitalizations in AI/AN children. Analyses are ongoing to evaluate the effectiveness of the maternal RSV (RSVpreF) vaccine in this population.
- Among AI/AN persons, mRNA COVID-19 vaccines were highly effective in preventing COVID-associated hospitalizations.
- AI/AN persons who were fully vaccinated against COVID-19 and those with milder COVID-19 illness were less likely to develop post-COVID conditions (or long COVID).
- During the first four years of the study, 2019-2024, around 10% of AI/AN children hospitalized with respiratory illness tested positive for influenza. Influenza-associated hospitalization rates were highest among children under 2 years old.
- During the first four years of the study, 2019-2024, around 13% of AI/AN children hospitalized with respiratory illness tested positive for HMPV. Younger age (<2 years old compared to 2-4 years old) and presenting to care 4 or more days after illness onset were associated with increased risk for HMPV-associated hospitalization.
- These data can be used by the Tribes to understand the burden of respiratory viruses and to guide use of prevention products to improve the health of the population.
To learn more about the results from this study, follow the links below:
- Respiratory syncytial virus (RSV)
- SARS-CoV-2 (the virus that causes COVID-19)
- Influenza
- Human metapneumovirus (HMPV)
We thank all study participants, IHS and tribal health facility medical staff, community members, the tribal ethical review boards, and the Centers for Disease Control and Prevention for making this study possible.
IRB #: NNHRRB NNR-19.350; PHX Area IRB 19.08; JHSPH IRB No. 00009605

